The corresponding pseudo bond graph for a two-component fluid accumulator is shown in Fig. 3. This pseudo bond graph expresses through its 0-junction structure the conservation of total mass, mass of each of the components and the total internal energy. The thermodynamic properties pressure P, mass fraction of each component cl, c2 and temperature T can unambiguously be calculated from the state variables mass m, mass of component two m2, volume V and total internal energy E. This basic model will act as the key building block for modeling of thermodynamic control volume systems, and obviously also for modeling of the diesel engine process to be introduced next.
Utilizing the bond graphs including the extended pseudo bond graph representation, consistent models for simulation of dynamic systems in general and diesel engine systems specifically can be developed with ease. This consistent representation of models from different energy domains is ideally suited for selection as a framework for describing models in a model library.
The advantageous features of the bond graph method for assembly and analysis of both the model and computational structure of the model, can be utilized to build structured large models without any hassle.
4. DIESEL ENGINE MODELS
The focus of engine simulations for transient performance is to define the mass, energy and work transients in each subsystem and the transient relations between the different subsystems. Describing the thermodynamic processes taking place in each of the subsystems following the working medium through the complete engine system is the common denominator of the engine models to be developed. Some basic assumptions for modeling of the thermodynamic process taking place are given next.
4.1 Thermodynamic modeling of engine processes
The working medium for the engine process consists of air plus exhaust gas produced from the combustion process. The combustion process is a chemical reaction which can be illustrated by
fuel + air → products (3)
The thermodynamic properties of the working medium is given by the compositions of the air and fuel, and the actual chemical process taking place. For calculation of the thermodynamic properties of the working medium it can be assumed that the composition of the fuel is given by CmHnOlNk, where the letters m, n, l and k are the average volume fraction of each component. This assumption covers all hydrocarbons and most natural gases.
The composition of dry air is available as standard admonisher, which contains numerous components in small amounts. For engineering applications a composition of oxygen and nitrogen are often found satisfactory. The humidity of the air can however play a significant role for the thermodynamic properties of the working medium, and must be included when specifying the composition of the air. In our routines [10] for calculation of thermodynamic properties the air can consist of a mixture of O2, N2, Ar, CO, CO2, H2O, H2.
The combustion reactions transforms fuel and air into products. The final composition of the products depend on the actual combustion process, and complete calculation of this process is impossible for most fuels. However, a simplified solution assuming that oxygen is available in excess so that the combustion is complete is often found to describe the gross results of the combustion process well. Using this assumption the composition of the products can be determined directly. For temperatures above 1700 K it is known that dissociation will contribute to the thermodynamic properties. The composition of the products can in this case be calculated using equilibrium considerations as in [11].
Assuming that the combustion products are a mixture of ideal gases, the thermodynamic properties can be expressed as functions of temperature, fuel-factor and pressure (only if dissociation occur), as shown in Table 1.
Table 1. Functions for thermodynamic properties for combustion products with and without dissociation.
where the fuel factor F is defined as the ratio of total amount of burnt fuel in the mixture to air and stoichiometric fuel/air ratio fs , and given by F = (mb /mair) / fs. The fuel factor completely specifies the composition of the mixture consisting of the two components burnt fuel mb and air mair.
In section 2 we introduced the pseudo bond graph model for multicomponent thermo-fluid systems using the mass fractions to describe the composition of the fluid. Using a slight modification a pseudo bond graph model for mixture of air and burnt fuel can be introduced using the fuel factor as the effort on the second bond, the mass flow of burnt fuel as the flow on the second bond and introducing burnt fuel as a state variable. This then gives the constitutive laws for the C-field model of a gas accumulator shown in Fig. 3 as
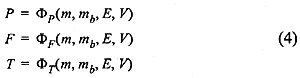
The actual relation for the fuel factor is given as
F=(mb/mair)/fs = (mb/(m-mb))/fs (5)
and the temperature and pressure are calculated using the thermodynamic relations introduced above in an iterative solution procedure.