|
IMPLICATION OF N: P: SI RATIOS TO HARMFUL ALGAL BLOOMS IN HONG KONG WATERS
K.C. Ho1, John Hodgkiss2 and Ironside Lam2
1School of Science and Technology, The Open University of Hong Kong
Hong Kong, CHINA
kcho@ouhk.edu.hk
2Department of Ecology & Biodiversity, The University of Hong Kong
Hong Kong, CHINA
ABSTRACT
Since the 1980s, waters in Hong Kong and Southern China have suffered from occasional attacks of harmful algal blooms (HABs). An episode in 1998 resulted in a total loss of 350 million Hong Kong dollars in the aquacultural industry. While most HABs were triggered by special oceanographic and climatic conditions, microalgal biomass was closely related to nutrient supplies. Past research showed that dinoflagellate-caused HABs were associated with changes in N:P (atomic) ratio when the minimum concentration of 0.1 mg-N/L of TIN and 0.02 mg-P/L of DIP was reached. Recent findings indicate that interspecific competition between diatoms and dinoflagellates should not be overlooked. Silicate (Si), the limiting factor for diatom growth, has played a significant role in determining the dominant species and the magnitude of red tides. The Redfield Ratio should be critically reviewed in terms of its application to HABs dominated by dinoflagellates and the Si:N ratio should be considered in parallel with N:P ratios. Data from Tolo Harbour of Hong Kong show that dinoflagellate blooms are favored by N:P (atomic) ratio of 10-22 and Si:N (atomic) ratio <1; diatom blooms are favored by larger N:P and Si:N ratios; whereas micro-flagellate blooms often occur after collapse of diatom and dinoflagellate blooms. Therefore, besides discharges of domestic sewage and agricultural wastes, which contain a huge quantity of TIN and DIP, variation of Si input due to urbanization, soil erosion and river diversion should be considered.
INTRODUCTION
Harmful algal blooms (HABs), which used to be known as red tide, are a global
concern. Like other temperate and subtropical waters. Hong Kong and indeed the whole southern portion
of China have been frequently affected by HABs since the 1980s. A total of 644 HAB incidents were recorded
in Hong Kong waters particularly Tolo Harbour from 1980 to 2001 ( Hong Kong
Red Tide Information Network. 2002). In addition, 69 HABs/red tides occurred in other South China
waters ( Hodgkiss et al., 2001).
Harmful algal blooms often result in extensive fishkills in aquacultural zones.
For example, a prolonged HAB in April - May 1998 caused around a 350 million Hong Kong dollars (〜42 million
US dollars) loss in the local aquacultural industry ( Lu and Hodgkiss, 1999).
While a great deal of resources have been deployed in forecasting as well as mitigating HABs, the major
formation mechanism and limiting factors have yet to be clarified by scientists. This paper presents analysis
of the observed characteristics of HABs in Hong Kong waters and this information is anticipated to be
useful in understanding the principal ecological processes of HABs in the coastal marine environment.
Figure 1. Hong Kong and the location of Tolo Harbour (hatched)
CHARACTERISTICS OF HABs IN TOLO HARBOUR
Of the various affected waters in Hong Kong, Tolo Harbour, a semi-enclosed
embayment with a narrow outlet channel in the northeast of Hong Kong (Fig.1), have been extensively studied
in terms of the causes and impacts of HABs ( Holmes and Lam, 1985; Hodgkiss
and Chan, 1987; Lam and Ho, 1989a and 1989b;
Wong, 1989; Lam and Yip, 1990; Hodgkiss
and Ho, 1992; Ho and Hodgkiss, 1993a and 1993b;
Hodgkiss and Ho, 1997). In addition to tidal force and oceanic intrusion of
water which carried vegetative cells into Tolo Harbour, salinity shock and the uniform meteorological
conditions (cool temperature, overcast skies and low rainfall) in March to early May every year were believed
to allow favorable growth of causative dinoflagellates during HABs ( Lam and Yip,
1990; Yung et al., 1997). While nutrients (N, P) and micro-nutrients (ferric
ions, Vitamins) are considered the major triggering and supporting factors of HAB ( HO
and Hodgkiss, 1991), interestingly, Holmes and Lam (1985) and Holmes (1988)
showed the positive correlation between nitrogen loading increase in the watershed of Tolo Harbour and
the increase in red tide incidents there. Extensive urban development, untreated domestic sewage and discharge
of livestock wastes were considered the major contributors to increase nitrogenous compounds and phosphates
in inner Tolo Harbour during the 1980s to early 1990s. Going one step further, Ho
and Hodgkiss (1993b) and Hodgkiss and Ho (1997) reported that when the
minimum concentration of 0.1 mg-N/L of TIN and 0.02 mg-P/L of DIP was reached, red tide occurrence in
Tolo Harbour was highly possible. Furthermore, Hodgkiss and Ho (1997) revealed
that the decline in annual mean N:P (atomic) ratio 1982 - 1989 in the surface water of Inner Tolo Harbour
was often associated with increase in red tide incidents in the same year (Fig.2A). Their conclusions
were generally agreed with the findings of Hodgkiss and Chan (1987), Chan
and Hodgkiss (1987) and Huang et al. (1994) that a decline in N:P ratio
in surface water usually resulted in dinoflagellates (the main red tide causative organisms) taking over
dominance from the diatoms. Thus, the decline of annual mean N:P (atomic) ratios from around 20:1 in 1981
to 11:1 in 1990 reflected the change in phytoplankton community in Inner Tolo Harbour during the 1980s
, when incidents of red tide peaked.
| (Enlarge: 40KB) |
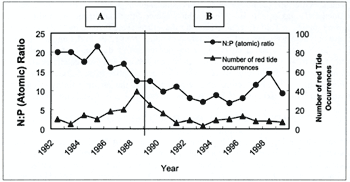 |
Figure 2. N:P ratios versus numbers of red tides in the surface waters of
Inner Tolo Harbour during the period A: 1982 - 1989 (after Hodgkiss and Ho, 1997) and B: 1990 - 1999 (updated
by the present authors)
Table 1. Optimal N:P (atomic) ratios in cultivated medium for various red-tide
causative dinoflagellates and diatoms in Tolo Harbour (After Hodgkiss and Ho, 1997)
| Species |
Optimal N:P (atomic) Ratio |
| Alexandrium catenella |
(15-30) : 1 |
| Ceratium furca |
(12-22) : 1 |
| Cryptomonas sp. |
(12-20) : 1 |
| Gonyaulax polygramma |
(4-8) : 1 |
| Karenia mikimotoi |
(11-16) : 1 |
| Noctiluca scintillans |
(8-14) : 1 |
| Olisthodiscus sp. |
(6-15) : 1 |
| Prorocentrum dentatum |
(6-13) : 1 |
| P. minimum |
(4-13) : 1 |
| P. sigmoides |
(4-15) : 1 |
| P. triestinum |
(8-15) : 1 |
| Prymnesium spp. |
(6-12) : 1 |
| Pseudonitzschia pungens |
9 : 1 |
| Scrippsiella trochoidea |
(6-13) : 1 |
| Skeletonema costatum |
>24 : 1 |
|
During the 1990s, the levels of TIN and DIP in the surface water of Inner Tolo
Harbour were significantly reduced due to enforcement actions under the Water Pollution Control Ordinance
of Hong Kong ( Environmental Protection Department 1991-2001). While
the frequency of red tide have also been reduced since 1991. Figure 2B shows that the association between
decreases in N:P (atomic) ratio and increases in red tide incidents as shown in Figure 2A is nearly no
changed. This confirms that red tide formation, which is mainly due to rapid dinoflagellates growth in
the surface water of Inner Tolo Harbour, demands minimum (critical) concentrations of TIN and DIP and
specific N:P (atomic) ratios. This conclusion is supported by bottle algal bioassay experiments (Hodgkiss
and Ho, 1997) which showed that out of the 15 red-tide causative organisms studied in Tolo Harbour, eleven
dinoflagellates were favored by N:P (atomic) ratios of 4-16 and only four were favored by N:P (atomic)
ratios from 12-30 in the initial culture medium (Table 1). For Skeletonema costatum, a red-tide
causative diatom, was favored by an N:P (atomic) ratio >24. The optimal N:P ratios in coastal diatoms
and dinoflagellates are found to be different from the Redfield Ratio of 16:1 which has been applied to
phytoplankton of open water ( Redfield et al., 1963). The discrepancy is
probably due to the adapting ability of phytoplankton to strong land-based discharge and salinity stress
in estuarine and coastal environment.
SIGNIFICANCE OF INTER-SPECIFIC COMPETITION
While the relationship between N:P (atomic) ratios and HAB occurrences has
been widely discussed, the related inter-specific competition within the phytoplankton community has not
yet been comprehensively elaborated ( HO and Hodgkiss, 1991). In fact, from
the above conclusions regarding the relationship between N:P ratios and red tide occurrences at Tolo Harbour,
it can be seen that diatoms and dinoflagellates are assumed to have a similar ecological niche in coastal
marine waters and their strive for a nutrient supply similar too. Hence, it is not unexpected that strong
biological competition occurs between diatoms, dinoflagellates and other small flagellates (including
zooplankton) in the community. This hypothesis is supported by Figure 3, which records the population
dynamics of diatoms, dinoflagellates and small flagellates during a red tide in Inner Tolo Harbour in
March 2000. During most times of the year, diatoms are the dominant group within phytoplankton in Inner
Tolo Harbour ( Chan and Hodgkiss, 1987; Lam and Ho,
1989b). Nevertheless, as seen in Figure 3, when the seed population of Noctiluca scintillans
began to bloom, the cell concentration of diatoms (dominated by Skeletonema costatum ) was significantly
reduced, with dominance taken over by dinoflagellates. While the population of N. scintillans changed
occasionally due to diurnal migration between surface and middle layers ( Blasco,
1979; Ho, 1994), after one to two days the red tide caused by N. scintillans
dissipated, and then, diatoms (mainly Skeletonema costatum ) resumed their dominance of the phytoplankton
community. When both dinoflagellate and diatoms blooms were over small flagellates assumed dominance for
a short period of time. A few days after this red tide event, diatoms resumed their normal dominance in
Inner Tolo Harbour.
On the basis of observations and studies, HABs cannot be viewed solely as a chemical response by specific species of phytoplankton to supply of nutrients. Although nutrients are able to trigger and support HABs, the fundamental changes in phytoplankton dynamics are closely related to biological processes and ecological competition. Therefore, it is likely that HABs are actually a succession process in the coastal marine environment, where the dominance by diatoms, dinoflagellates and small flagellates (including zooplankton) is gradually taken over by each in turn.
| (Enlarge: 30KB) |
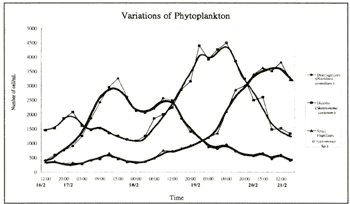 |
Figure 3. Changes in the diatom, dinoflagellate and small flagellate populations
in Inner Tolo Harbour during a red tide in February 2000
It has been reported that dinoflagellates have a relatively slow growth rate,
rarely exceeded one doubling per day ( Karentz, 1983). Since diatoms are
able to have 2-6 doublings per day, this can result in prolonged dominance of diatoms in marine and estuarine
environments ( Werner, 1977; Stoermer and Smol, 1999).
As observed in Tolo Harbour, only when the available nutrients or environmental conditions changed, dinoflagellates
take over the dominance ( Lam and Ho 1989b). Thus, when the surface nutrient
supply dwindled, diatoms receded in importance and dinoflagellates took up dominance because of their
migrating ability between surface and middle layers and their different requirement for nutrients and
N:P ratios ( HO and Hodgkiss, 1991; Ho, 1994). Further
studies on phytoplankton dynamics in relation to competition at genus and species levels should be considered
in future HAB research. |