Table 6 - NAMO results with overbased saliclates
In addition some salicylate samples were blended in 650N/115N basestocks. The corresponding NAMO results are shown in Table 7. They have to be compared to those of Table 6.

Table 7 - NAMO results with overbased salicylates diluted in 650N/115N basestocks

Table 8 - NAMO results with PIB-succinimides
4. DISCUSSION
The neutralization ability of the various types of additives can be compared with the data obtained. The effect of the composition and the influence of the manufacturing process are shown. The effect of the basestocks and of other surfactants, such as PIB-succinimides, is also clear.
4.1 - Conrparison of additive types
The various types of overbased additives of the same BN range can be compared.
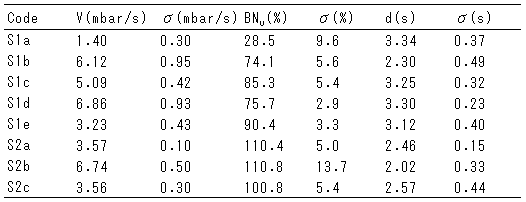
The data obtained for the samples Sle (salicylate), PH1 and PH2 (phenates) and A4a, B4 and C4 (sulfonates) show a significant variation of the neutralization speed - see Fig. 4. The initiation delay, d, is shorter for the sulfonate type additives than for the others although it is not significantly different from phenate to salicylate type additives. The BN used does not seem to follow the same variation and is not clearly correlated to the chemistry of the detergent. This fact is logical as BNu depends on the CaC03 content and as the BN is brought for a part by organic functions in the case of phenate and salicylate type additives.
In conclusion, overbased sulfonates can be considered as more reactive than overbased phenates and salicylates. Their neutralization speed is, on average, 60% higher than the speed of the salicylates and 42% higher than the speed of the phenates.
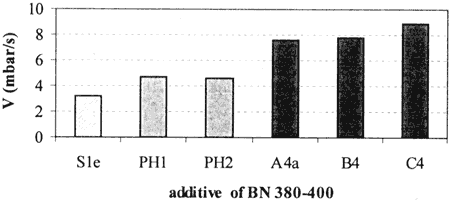
Fig. 4 - Neutralizatron speed vs type of additive
4.2 -Overbased sulfonates
The various samples of overbased sulfonates tested allow one to observe the effect of many parameters related to the additive chemistry such as the BN level, the ratio limelCaCO3, the metal salt nature and form, the soap content. the surfactant type and the alkylate chemistry. The effect of the catalyst used to make the alkylates, basic molecules of the overbased sulfonates, and the effect of a particular additive used to enhance the carbonation during the overbasing process are also observed.
4.2.1 - BN effect and soap availability effect
The samples A3a, A4a and A5 have the same surfactant system and were prepared by using the same process. They vary by their BN Ievel, which is respectively in the range of 300, 400 and 500, and by their soap content, which is respectively of 28%, 19% and 20%.
As it can be seen on Fig. 5, the neutralization speed, V, increases with the BN from 300 to 400 and then decreases from 400 to 500. This result is surprising, as one would believe that V might have increased proportionally to the BN level. This means that the BN Ievel itself is not the only parameter that influences V.
It has been previously shown [5] that the kinetics of the reaction depends on the transportation ofthe species in the oil film by means of the surfactants available. Therefore, as the data do not show a direct correlation between V and the soap (surfactant) content, it can be supposed that both the CaCO3 (BN) and the soap availability have a non-independent effect on V. This is confirmed when comparing the speed of A3a. made by a direct overbasing process, and the speed of A3b, made by blending A4a with a neutral sulfonate. In A3b "free" soap is available while in A3a the soap is tied up.
Fig. 5 - Neutralization speed vs BN level
BACK CONTENTS NEXT