Table 1 Principal Particulars of Engines
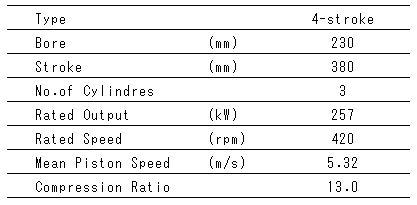
Table 2 Properties of Fuel
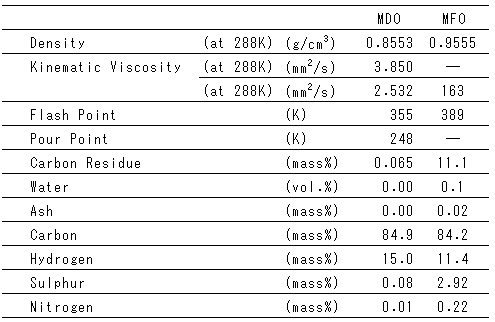
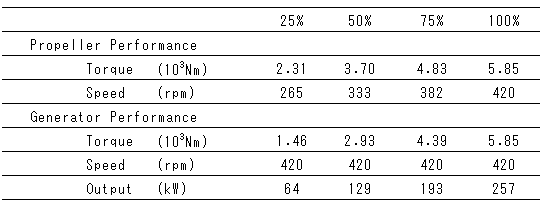
Table 3 Engine Operating Conditions
2.2 Chemical Analysis of PM
In general, the components of PM are mainly distinguished into soot, soluble organic fraction (SOF), sulphate and binding water. SOF consists of organic compounds that are soluble in specific organic solvents. The other components except SOF are also called insoluble fraction (ISF) in a lump. Sulphate noted in this context would contain metal sulphates as well as sulphuric acid. Binding water is captured by sulphate ion in PM. If PM is conditioned at 298K and relative humidity of 50% in a conditioning chamber for 24 hours similar to this experiment, the mass of binding water is obtained by multiplying the mass of sulphate ion by 1.3 shown by Wall et al. [4]
In this study, SOF was separated by Soxhlet extraction at 333K for 16 hours with 100ml of dichloromethane (CH2CI2) from PM sampled on one pair of filters to be weighed. In some cases, it was analyzed by gas chromatography (GC; Shimadzu: GC-7AG) and gas chromatograph mass spectrometry (GC-MS; Shimadzu: GCMS-QP1000) to investigate the detailed chemical aspects of SOF. Moreover, sulphate ion was separated from the residue on the sampling filters by supersonic extraction and analyzed by ion chromatography (IC: Dionex: 2000i/SP) to measure sulphate in ISF. These analytical conditions are shown in Table 4.
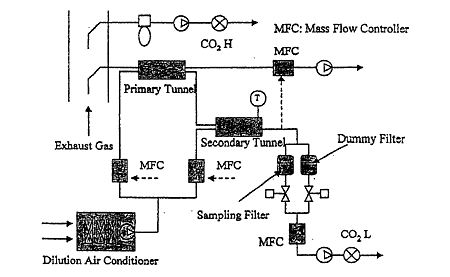
Fig.1 Schematic Diagram of Double Dilution Tunnel System
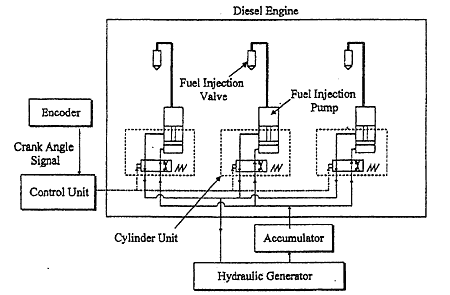
Fig.2 Schematic Diagram of Hydraulic Drive Fuel Injection System
3. RESULTS AND DISCUSSION
3.1 Basic Emission Characteristics and Chemical Aspects
At first, the basic emission characteristics of PM from the marine Diesel engine are described. The results of measurement of PM emission characteristics are shown in Fig.3 with SOF/PM ratio. SOF emission rate would be obtained by multiplying the mass of PM by SOF/PM ratio provided by Soxhlet extraction.
BACK CONTENTS NEXT