5. CONCLUSIONS
It is very important to investigate accurately the behavior of seawater components in order to water quality control of marine boilers, and also the behavior of Mg2+, especially when OH- and PO43- coexist with it, and when SiO2 is mixed. A series of our studies revealed that there is a limitation of the applicability of the conventional chemical reaction model of scale components and water treatment chemicals of phosphate/alkali type. This paper proposes, therefore, a new model of chemical reaction system as follows.
1] 10Ca(HCO3)2+6Na3PO4+2NaOH→[Ca3(PO4)2]3・Ca(OH)2+10Na2CO3+10CO2+10H2O
2] 10CaCl2+6Na3PO4+2NaOH→[Ca3 (PO4)2]3・Ca(OH)2+20NaCl
3] Mg(HCO3)2+SiO2+2NaOH→MgSiO3+Na2CO3+C02+2H2O
4] (3n+1) Mg (HCO3)2+2nNa3PO4+2NaOH→[Mg3(PO4)2]nMg(OH)2+ (3n+1) Na2CO3+ (3n+1)CO2+ (3n+1) H2O
5] MgCl2+SiO2+2NaOH→MgSiO3+2NaCl+H2O
6] (3n+1)MgCl2+2nNa3PO4+2NaOH→
[Mg3(PO4)2]n・Mg(OH)2+ (6n+2)NaCl
7] SiO2+2NaOH→Na2SiO3+H2O
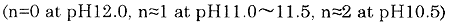
Though a new treatment method using synthetic polymers is now developing, treatment using the phosphate/alkali type chemicals would be required on board still the future. If so, the following cases should be taken into consideration in order to develop the automatic control of water quality in marine auxiliary boilers by monitoring of pH and EC.
1] A periodic manual check of PO43- in the boiler water is required because it is impossible to control PO43- automatically when hardness components and SiO2 coexist.
2] For a full-automatic control of water quality, it is required to introduce an apparatus such as demineralization treatment system in addition to the chemical treatment. The apparatus is for cleaning up hardness components of Ca, Mg, and chemicals of alkali type are for dissolving SiO2 into boiler water of high alkalinity.
3] Concentration of boiler water is controlled by monitoring of EC and injection rate of chemicals is controlled by monitoring of pH, and sampling flow velocity has to be adjusted properly so as to avoid the influence of streaming potential.
[Acknowledgement]
The authors should like to acknowledge sincerely the Kurita Water Industries Company for its considerable assistance in the analysis of the boiler water.
[References]
[1] Itami, Y., Nishikawa, E., et al., J, of MESJ, Vol. 28, No. 6 (1993), P356 (in Japanese).
[2] Itami, Y., Nis-hikawa, E., et, al., J. of MESJ, Vol. 30, No. 5 (1995), P405 (in Japanese).
[3] Itami, Y., Nishikawa, E., et, al., J. of MESJ, Vol. 33, No. 10 (1998), P759 (in Japanese).
[4] "Chemicals hand book", Kurita Kogyo company Ltd., (1988), p65 (in Japanese).
[5] "Water quality control of Boiler", Japan Boiler Association, (1984), p245 (in Japanese).
[6] James W. M., "The Chemical Treatment of Boiler Water" (1984), p17,Chemical Publishing Co.
[7] Itami, Y., Nishikawa, E., et, al., J. of MESJ, Vol. 34, No. 10, (1999), P667 (in Japanese).