The principle is fairly easy and just involves a mixing vessel where liquid carbon dioxide is sent through a pipe and mixed with seawater at about 500 m depth.
Aya et. al. have proposed yet another approach for self-sinking of carbon dioxide (Aya et. al. (1999)). In this approach, denoted as COSMOS, cold CO2 at -55 degrees Celsius is sent through a pipeline to around 500 m depth and the deposited through a nozzle system that produces a droplet of around 1 m in diameter. Preliminary estimates indicate that the this droplet size might be necessary for the droplet to reach 3000 m fast enough to keep a density heavier than seawater (heating from the surroundings). The temperature at deposition depth is estimated to be between -40 and -35 degrees Celsius. Part of the strategy in this concept is that the large temperature difference may induce rapid ice growth on the surface of the carbon dioxide droplet.
In this context we consider the approach of Kobayashi as an intermediate ocean sequestration and focus on the three approaches where the main issue is to use gravity for transport of encapsulated carbon dioxide to large depths. Solid carbon dioxide and carbon dioxide hydrate will both have the necessary mechanical strength to withstand pressures up to 280 bars without collapsing. The issue of stability of these two categories of solid substance is therefore a purely thermodynamic one.
The dry ice alternative is far too expensive to be of any practical interest due to the cost of freezing of carbon dioxide. In this context we will therefore not discuss this option explicitly. Some aspects of the ice freezing on a cold surface (below freezing temperature of seawater), as discussed for a sub-cooled C02 liquid droplet, will however also apply to cold dry ice and will contribute to a reduction in the melting rate of the dry ice during it's sinking towards the bottom.
The COSMOS approach is a more complex issue than hydrate or dry ice. The thermodynamics, heat transfer kinetics and ice formation kinetics are implicitly related to frictional forces as well as static pressure that will tend to break the droplet during it's transport towards the bottom. This is the subject of an ongoing experimental and modeling study under a project financed by NEDO (Kvamme et. al. (2000)). In the context of this paper we will limit the discussion to the thermodynamic and kinetics of the most important phase transitions involved.
In section II we discuss the differences in the thermodynamic driving forces for destabilization of the three types of sending strategies. In section III we discuss briefly the corresponding kinetics involved. Section IV describes qualitatively some of the challenges related to the COSMOS sending method.
II. THERMODYNAMIC STABILITY OF CARBON DIOXIDE HYDRATE
Combination of the first and second law of thermodynamics states that a system will always stribe to reach a minimum in Gibbs free energy under the effects of pressure, temperature and exchange of mass. As a result of this any stable thermodynamic state involving more than one phase implies that all phases must have the same temperature and pressure. In addition each component in the coexisting phases must have the same chemical potential.
I consider a total system of seawater and carbon dioxide encapsulated. When producing the hydrate the components are in three different phases, liquid water, hydrate and carbon dioxide. The chemical potential for water in the liquid water phase may be written as:
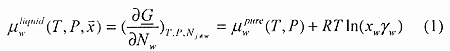
where T is absolute temperature in Kelvin, P is pressure, x is mole fraction, γ is activity coefficient and subscript w denote water. The line under the G (for Gibbs free energy) indicates extensive property (Joule). R is the universal gas constant. Pure liquid water chemical potential as calculated from the TIP4P model is systemized in table 10 of Kvamme and Tanaka (1995). These chemical potentials are for 1 bar pressure.