|
6 INFORMATION RELATED WITH TOXICITY EVALUATION
6.1 Toxicity to aquatic organisms
6.1.1 Acute toxicity
We researched the United States EPA database (ECOTOX) to obtain data on the acute toxicity to aquatic organisms.
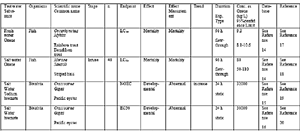
Table 8 shows the acute toxicity of the Ozone used as an Active Substance for this system and bromate ion, a relevant chemical for the treatment of seawater. Data in Table 8 is extracted from ECOTOX, which shows the end points clearly and the lowest values, that stands for strong toxicity. These data is based on analysis of oxidant concentration in the water (refer to "section 4. Analysis Method for Active Substances and Relevant chemical" for the analysis method).
Acute toxicity of Ozone (LC50) is 8.1μg/L in the fresh water and 50μg/L in the seawater.
Acute toxicity of bromate ion is 32,000μg/L, which is required by NOEC (as sodium bromate ion) against Crassostrea gigas.
As described above, acute toxicity of Ozone is very strong both in fresh water and seawater. On the other hand, the acute toxicity of bromate ion in this system about which influence at the time of discharge of ballast water is apprehended, is relatively weak, about 1/1000 of that of Ozone.
Table 8. Acute aquatic toxicity
| (拡大画面:31KB) |
 |
|
6.1.2 Chronic toxicity
There exists no data on the chronic toxicity of Ozone and bromate ion but both possess acute toxicity.
Ozone has a half-life period of 5.8 seconds in the seawater and is supposed to be depredated in 30 minutes in the same way in the fresh water also. Ozone feeding concentration for treating the ballast water of this system (at the full ballast water capacity) is designed to be 4 mg/L or below. Based on the decomposition speed as described above, when the ballast water is discharged, the Ozone is believed to decompose to the concentration level of 0.14μg/L (refer to paragraph 6.3.7) which may exist in the environmental water. Thus, it is supposed that the Ozone has no chronic toxicity.
Half-life period of bromate ion in the seawater is about 12 hours relatively longer than Ozone. Though the acute toxicity is relatively weak, about 1/1000 of that of Ozone, chronic toxicity of ballast water discharged is apprehended. Therefore, a chronic toxicity test of the bromate ion was conducted on the seawater organisms. Method and results of this test me described in "section 8. Toxicity testing of the treated Ballast Water".
6.1.3 Endocrine disruptive properties
No instance has been reported on the endocrine disruptive properties for both Ozone and bromate ion (as a result of research in the ECOTOX database). Because of the dissolution speed, it is considered that there is no internal disturbance.
6.1.4 Toxicity of sediment
Soil absorption factor of bromate ion is estimated as follows from the relation with the octanol/water partition coefficient, Pow:
Koc=0.41xlog Pow(-7.18)8)=2.7x 10-8L/kg
This estimate of Koc: 2.7x10-8L/kg is far below the value of anticipated toxicity to benthic organisms and, therefore, there is no anxiety for the toxicity to benthic organisms.
Decomposition speed of Ozone is higher than that of bromate ion and its absorption into the soil is not anticipated and there is no fear of toxicity to benthic organisms.
6.1.5 Biomagnification/bioaccumulation
Octanol/water partition coefficient log value of bromate ion, logPow, is -7.18 and is estimated to be free from fear of concentration to organisms.
Ozone decomposition speed is far higher than that of bromate ion and the Ozone is promptly decomposed to oxygen and water in the water. Therefore, there is no fear that the Ozone may be concentrated in organisms.
6.2 Mammalian toxicity
6.2.1 Mechanism of toxicity to mammal
Toxicity of Ozone and bromate ion to mammal is based on its strong oxidizing property.
Ozone exerts various influences on the mammal such as irritation to eyes, or to pharynx → trachea → bronchus → bronchiole → cells caused by inhalation.
Bromate ion also has various effects on the skin and eyes by exposure, and cough, pharynx pain, nausea, vomiting, abdominal pain, diarrhoea, or central nervous system caused by taking orally.
6.2.2 Effects on skin and eves
Ozone gives no effects to the skin but causes irritation to eyes when exposed to the Ozone of 2ppm or more for several minutes22. Bromate ion exerts effects on both the skin and eyes so that the skin flares and eyes flare and pain23.
6.2.3 Acute toxicity
Acute toxicity of Ozone (4 hours. LD50) to the mouse is 5.9〜6.8ppm22).
Acute toxicity of bromate ion to hamsters is reported to be 280〜495mg/kg of LD50 when taken orally25.
6.2.4 Chromic toxicity
When Ozone is sucked for a long time, it gives effects on the lungs of human and mammal even if the concentration is extremely 1ow22.
In tests of bromate ion for rats for 15 weeks, NOAEL:30mg/kg/day is required25.
6.2.5 Carcinogenicity
No information has not been acknowledged on the carcinogenicity of Ozone25.
As for the bromate ion, there has been an example of a test of oral dose to mice and rats for 100 weeks and carcinogenicity to the kidney, etc., have been recognized24.
6.2.6 Mutagenicity
Most of research reports have denied the mutagenicity of Ozone24.
Tests have been conducted on salmonella or colon bacillus of bromate ion and shown negative results24.
6.2.7 Secondary toxicity and toxicity of secondary chemicals
As described in section 3, secondary chemicals other than the bromate ion can be actually ignored. Therefore, it is considered that toxicity of secondary products other than bromate ion may be ignored.
6.3 Environmental fate and effect under aerobic and anaerobic conditions
6.3.1 Decomposition process
Ozone has high self-decomposition. The Ozone molecule itself is basically an unstable substance and its decomposition is prompted promoted under high temperature and high pH in the water. It is finally decomposed to oxygen and water in the water. When there are organisms, Ozone reacts to them to oxidize. By the decomposition reaction of organisms, Ozone is decomposed and consumed (see section 3 for details).
Bromate ion is decomposed by the action of anaerobic bacteria such as methanogen in anaerobic situation.
6.3.4 Potential effects on wildlife and benthic habitats
As the half-life period of gaseous phase Ozone is as long as several to several tens of hours, its effects on organisms exposed to it is apprehended. The half-life period of Ozone is 5.8 seconds in the seawater and the Ozone is decomposed in about 30 minutes in the fresh water. Therefore, it is considered that the Ozone has no potential effects in the water.
Effects of bromate ion, a relevant chemical produced in the seawater, is also apprehended. However, since its half-life period in the seawater of bromate ion is fairly short as about 12 hours and the estimated absorption coefficient is as very low as 2.7x10-8L/kg, there is no potential of effects on benthic organisms. It is considered that there is no such effects either as bromate ion may be accumulated in high-order organisms through a food chain.
6.3.5 Potential residues in seafood
Both the Ozone and bromate ion are not accumulated, it is judged that there will be no potential residues in seafood.
6.3.6 Any known interactive effects
Ozone produces oxygen when decomposed in the water. As a result, it increases the concentration of oxygen dissolved in the water and helps to form the environment suitable for the aquatic organisms to inhabit. In the dosed seawater for aquaculture, Ozone has effect to remove ammonia nitrogen discharged from the fish.
Bromate ion is hard to be reduced in the water under the normal environmental condition but can be reduced to bromide ion where ferrous ion27, activated carbon or other carbonaceous substances exist28.
6.3.7 Distribution of Ozone in environment components (atmosphere, water, soil, sediment, suspended solid or organisms)
Since the Ozone decomposes quickly, it does not exist in the water or soil. It is produced by photochemical reaction, however, and there is extremely small amount of Ozone as a background value m the atmosphere. The background value in the atmosphere is about 200ppb at the maximum.
Ozone dissolves in the water basically according to Henry's law. On the hypothesis that the quantity of Ozone gas is 100%, possible dissolved concentration calculated based on Henry's law is 0.14x10-6 (g/L) as dissolved Ozone in the case of fresh water and as BrO3 in the case of seawater. That is, the concentration level in the environmental water is under the ppb order far below the analysis accuracy of the analysis method.
|