|
1.6 Entrainment/Dissolution from Surface Slick to Water Column
The entrainment or dispersion into the water column of substances other than oil which float at the water surface follows the same approach used in earlier Type A models (Reed, 1989), wherein the dispersion rate per second is
Da = K3 (W + 1)2/(1 + 50μ1/2δSt) 7 (3-5)
with the wind speed, W, expressed in m/sec here (Mackay et al., 1980). K3 is a constant, μ is viscosity (centipoise), δ is slick thickness (m) and st is interfacial tension between the pollutant and water. K3 is set equal to 0.11. In the case of hazardous substances which form a surface slick, Equation (3-5) appears to underestimate entrainment as compared to evaporation. Results developed by Wolff and Poels (1986) have been used to apply a correction factor Db which depends on solubility:
Db = K4(S/MW)0.2 8 (3-6)
where
S = solubility (mg/l)
MW = molecular weight (gm/mole)
K 4 = constant ( 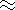 100)
For hazardous substances which float, the entrainment rate is then DaDb. Entrained substances are assumed to dissolve, as long as saturation conditions are not exceeded.
For oils and petroleum products, entrainment and dissolution modeling is based on the laboratory results of Delvigne and Sweeney (1988). The oil is represented by a large number of particles, each of which represents in turn a group of oil droplets of like size and composition (Elliott, 1986; Johansen, 1985). When a particle is on the surface, it behaves as a slick; when in the water column, a particle behaves as a droplet. The extent of the surface slick at any time is the superposition of all the surface particles at that time.
Transport of oil at the surface is computed simply as 3.6% of the wind speed, plus the current (Reed et al., 1990). Horizontal transport in the water column is computed from the current alone.
The entrainment of oil from the sea surface into the water column is based on Delvigne and Sweeney (1988):
Qdi = C* D0.57 S F di0.7 Δd (3-7)
where
Qdi = Entrainment rate per unit surface area, of oil droplets with diameters in the range ± Δd around di (kg/m2 sec)
C* = empirically derived entrainment coefficient
D = dissipation wave energy (kg/sec2)
S = fraction of sea surface covered by oil
F = fraction of sea surface covered by breaking waves per unit time (sec-1)
di = mean diameter of particles in size class i (m)
Δd = particle diameter interval (m)
The empirical coefficient C* is a function of the viscosity of the oil. Values for C* are given by Delvigne and Sweeney (1988) for three oils (Table 3.1).
|
Table 3.1
|
Values of the entrainment coefficient C* and the associated viscosity for three oils (Delvigne and Sweeney, 1988).
|
|
|
Fresh Prudhoe Bay Crude |
Prudhoe Bay Crude Weathered 10 days |
Ekofisk Crude |
|
|
|
|
|
| Viscosity v at 20℃ (10-6 m2/sec, Cst) |
|
92 |
220 |
8 |
| C* |
|
840 ± 200 |
510 ± 130 |
1800 ± 450 |
|
The limited data for C* suggests the approximation
C* = 4450 v-0.4 (3-8)
where
v = kinematic viscosity (10-6 m2/sec, Cst),
The dissipation wave energy, D, in kg/s2 is approximated from the breaking wave height, Hb in m, as
D = 0.0034 ρw g Hb2 (3-9)
where
ρw = density of seawater (kg/m3),
g = gravitational acceleration (m/sec2).
The fraction of the sea surface covered by breaking waves per unit time is approximated as
F = 3 x 10-6 W3.5 (3-10)
for the wind speed W in m/sec (O'Muircheartaigh and Monahan, 1986).
Observed droplet sizes range from 1 to about 1600 μm, with the majority of the oil in the range 50-350 μm. The droplet size distribution was shown by Delvigne and Sweeney (1987) to be related to kinematic viscosity, v, and the energy dissipation rate, e (J/m3 sec), by
d0 = C0 v0.34 e-0.4 (3-11)
According to Delvigne and Sweeney, the turbulent energy dissipation rate in breaking waves is on the order of 103 to 104 J/m3/sec; here we use an intermediate value of 5000. For the mean particle size, d50, the value of C0 is approximately 1400; for the maximum and minimum particle sizes, the coefficient is about 3400 and 500, respectively.
The initial entrainment depth is taken as
h0 = 1.5 Hb. (3-12)
A terminal rise velocity for entrained oil droplets of size class i is given by
wi = di2g (1 - ρ0/ρw)/18 vw (3-13)
where
ρ0, ρw = density of oil and water, respectively;
vw = kinematic viscosity of seawater.
The mixing depth, hi, for each particle size class i is taken as
hi = max (Dv/wi ,h0). (3-14)
The vertical dispersion coefficient, Dv, in m2/sec is estimated from data reported by Thorpe (1984), based on acoustic studies of air bubbles, as
Dv = 0.0015 W10 (3-15)
for the wind speed at 10 m height in m/sec.
The mixing depth for each particle class is allowed to increase to a maximum of 25 m, the observed depth of the pycnocline during these experimental spills. The model assumes uniform distribution of each particle size class over the associated mixing depth, so the resurfacing rate for size class i, mixed over the depth hi, is simply
Vi = (wi/hi) Δt. (3-16)
Laboratory flume studies (Delvigne et al., 1987) have shown that the majority of oil droplets formed in breaking wave conditions are in the range 50 ≦ d0 ≦ 350 μm. Table 3.2 suggests that, except for highly emulsified, high viscosity oils which tend to form larger particle sizes (Equation 3-11), most oil entrained in breaking waves will tend to remain subsurface as long as breaking continues to occur at virtually every location at least once per unit rise time. As soon as seas subside, however, any subsurface oil particles over 100 μm in diameter will resurface within an hour, reforming a surface slick.
|
Table 3.2
|
Characteristic parameters for oil droplets entrained in the water column, assuming a wave height of 1.3 m, a specific gravity for oil of 0.9, a turbulent dispersion coefficient Dv of 10-3, and an initial mixing depth of zm of 2 m.
|
Particle Diameter do
(μm) |
Rise Velocity (m/sec) |
Rise Time (zm/w) |
Steady State Time (Dv/W2) |
| 10 |
5.7 x 10-6 |
97 hr |
1 year |
| 50 |
1.4 x 10-4 |
4 hr |
14 hr |
| 100 |
5.7 x 10-4 |
1 hr |
0.9 hr |
| 300 |
0.005 |
7 min |
40 sec |
| 600 |
0.02 |
2 min |
2.5 sec |
| 1200 |
0.09 |
20 sec |
0.1 sec |
|
The viscosity μ is allowed to increase for petroleum products according to an emulsification algorithm, also from Mackay et al. (1980). The rate of incorporation of water into the slick is
Fwc = fraction of water-in-oil
W = wind speed (m/sec)
Gasoline, kerosene, and light diesel fuel are assumed not to form emulsions with water (Payne and Phillips, 1985). The resultant viscosity μ of the oil in the slick is then computed using the Mooney (1951) equation
μ/μoexp(2.5Fwc/(1.0-0.65Fwc)) 10 (3-18)
in which μo is the viscosity of the parent oil connected for temperature and evaporated fraction. The effect of evaporation on viscosity is modeled
as
μ = μoexp(C4Fevap)11 (3-19)
where Fevap is the fraction evaporated from the slick. The model uses C4 = 1 for gasoline, kerosene, and light diesel fuel, and C4 = 10 for other petroleum products. (Mackay et al., 1982).
The entrainment process is assumed to affect all four modeled fractions of a petroleum product equally. Aromatics are modeled as dissolving from entrained oil droplets and surface slicks using a standard mass-transfer and differential concentration equation (Equation 3-25).
Entrained "residual" oil is assumed to exist as particulate oil in the water column, and does not enter the toxicological concentration computations. This dispersion/emulsification formulation will eventually drive all residual oil into the water column, giving surface slicks a finite lifetime.
|