|
METAMORPHOSIS INDUCTION AND ITS POSSIBLE APPLICATION TO CORAL SEEDLINGS PRODUCTION
Masayuki Hatta1 and Kenji Iwao2
1Department of Biology, Ochanomizu University
Bunkyo-ku, Tokyo, JAPAN
mhatta@cc.ocha.ac.jp
2Akajima Marine Science Laboratory
Zamami-son, Okinawa, JAPAN
ABSTRACT
Coral reefs are the bases of ecosystems and environments in tropical and sub-tropical shallow seas; however, despite their importance, coral reefs around the world are declining at an alarming pace. In Indo-Pacific reefs, the coral genus Acropora consists of major components, and coral communities are maintained in large part by recruitment of their larvae. Many acroporids participate in 'mass spawning' in which a large number of species spawn their gametes synchronously. Huge numbers of larvae are produced by mass spawning, but most of them are lost prior to metamorphosis and settlement. If the larvae were collected and grown into colonies under controlled conditions, they could be used as donors for transplantation without damaging existing coral communities. Previously we found that a hydra neuropeptides induced the metamorphosis and settlement of Acropora larvae with 100% efficiency. Using the peptide, we succeeded producing primary polyps from coral larvae collected in the field. The primary polyps could be used for transplantation with the aim of reconstructing denuded reefs. Here we propose 'coral seedlings production' by collecting larvae after mass spawning and controlling metamorphosis with the peptide.
INTRODUCTION
Coral reefs are the bases of ecosystems and environments in tropical and sub-tropical
shallow seas. Despite their importance, coral reefs all over the world are declining at an alarming pace
as a consequence of direct and indirect human activities even though preservation and management measures
have been taken. The conspicuous reef decrease is due to coral bleaching, which is the loss of color in
corals in appearance as corals' intracellular symbiotic algae are lost. Coral bleaching has increased
in frequency, intensity and extent over the last two decades ( Huppert and Stone,
1998). Most recently, in the summer of 1998, high sea surface temperatures (SSTs) resulted in massive
mortality of reefs worldwide ( Tsuchiya, 1999). Many shallow reefs that
have been impacted are dominated by the genus Acropora, since this genus consists of major components
of the reefs in Indo-Pacific oceans and acroporids are sensitive to high temperature. Some reefs are recovering;
e.g. around Kerama Islands in Okinawa, Japan. Others, however, remain denuded such as those around Okinawa's
main island. Active restoration is acquired in Okinawa and in many other areas. Since coral communities
are maintained in large part by recruitment of their larvae, increasing recruitment efficiency of acroporids
through human intervention would be an effective contribution to reef restoration.
Many acroporids reproduce sexually in a unique manner termed 'mass spawning';
huge numbers of gametes are released into the water column synchronously within an hour by many colonies
belonging to multiple species ( Babcock et al., 1986; Hayashibara
et al., 1993). The buoyant gametes form high-density patches called 'slicks' on the sea surface. The
resultant larvae are dispersed by currents and settle on substrates to start their sedentary life. Despite
a large number of larvae being produced by mass spawning, most of the larvae are lost during the drifting
period and only a small fraction are recruited into reef communities. If such larvae that will be lost
prior to settlement are collected and grown to colonies under controlled conditions, they could be used
as donors for transplantation without damaging existing coral communities. The problem to date has been
the lack of a method by which to control metamorphosis and settlement of Acropora. Recently, we
found that one of hydra neuropeptides can induce metamorphosis of acroporids' larvae into polyps at high
rates ( Iwao et al., 2002). In this study, we tested the use of the peptide
in 'coral seedlings production', which consists of collecting coral larvae after mass spawning events
and producing primary polyps or infant colonies for the purpose of transplantation.
MATERIALS & METHODS
Preparation and maintenance of coral larvae
All experiments were done at Aka Island of Kerama Islands in Okinawa, Japan. Colonies of Acropora tenuis were collected two days before the predicted spawning date and kept in the Aka fishery harbor. When gamete bundles emerged at the mouth of the polyps, each colony was put in a separate bucket, and allowed to spawn in the laboratory. The egg-sperm bundles taken from five colonies were mixed in a bowl for two hours to allow fertilization, and then the eggs were transferred to new bowls filled with fresh 10-micron-filtered seawater. Larvae were transferred to new bowls with daily changes of filtered seawater, and maintained in densities of 1000-3000 per bowl (about 1.5 liter seawater, 25 cm diameter) at 26℃elsius.
Slicks of coral embryos/larvae were scooped in the Aka port in the morning at 9 - 10 a.m. after the mass spawning event in the night on May 25, 2000, and larvae were maintained as above.
Metamorphosis assay
Planula larvae were once washed with seawater filtered through a 0.2-micron
nitrocellulose filter (filtered seawater, FSW), and 10 larvae were placed in each well of 24-well culture
multiplates (SUMILON) with 1 ml of FSW. The peptide Hym-248 ( EPLPIGL Wamide,
Takahashi et al., 1997) was added to the indicated final concentrations, and the number of metamorphosed
animals, which formed mesenteries (Fig.1B) was counted 12 hours after the beginning of the treatment.
All experiments were done at 26℃elsius.
RESULTS
Morphological changes during metamorphosis induced with the peptide
Figure 1 shows a representative of morphological changes during metamorphosis of Acropora tenuis, induced with 1x10-6M Hym-248. Panel A is the planula larva swimming to the aboral direction with cilia. The larva attached the aboral side to the culture dish and contracted subsequently, after 1hr of the peptide treatment. After four hours, the aboral side transformed to a stalk-like structure, which soon shortened, and septation of the tissues became visible. The mouth became obvious within six hours. The whole tissue flattened, and twelve mesenteries were clearly formed after 12 hours (panel B). Panel C represents a primary polyp three days after the peptide treatment. Tentacles and calcified septa were formed. The metamorphosis processes appeared normal, since no morphological differences were noted between animals induced with the peptide or calcareous algae and those metamorphosed incidentally in bulk cultures (data not shown).
Figure 1. Morphological changes during metamorphosis
Panel A represents a side view of a planula larva of A. tenuis. The oral and aboral ends of the planula are indicated by 'o' and 'ab' respectively. Panel B and C show polyps after metamorphosis, viewed from the top.
Time requirement for metamorphosis commitment
To examine the minimal time required for the Hym-248 treatment to induce commitment
to metamorphosis, the peptide-containing FSW was replaced by fresh FSW after various interval times, and
the frequency of metamorphosis was counted at 12 hours after the onset of the peptide treatment. The results
using A. tenuis larvae 10 days after fertilization are shown in Figure 2. The larvae rounded up
after one hour, but regressed to normal planulae when the peptide-FSW was replaced by FSW within three
hours. Significant rates of metamorphosis were observed when the larvae were incubated with the peptide
for more than four hours. The relatively lower rates of metamorphosis were obtained in this particular
experiment compared to simple induction experiments, which gave rise to mostly 100% metamorphosis ( Iwao
et al., 2002, also see below) probably due to extensive washing, which may have caused physiological
stress. But the results clearly showed a gap between three and four hours. Thus, we conclude that a four
hours' exposure to Hym-248 is the minimum required for irreversible commitment to the metamorphosis pathway
at 26℃, hence the minimum treatment time for metamorphosis induction. 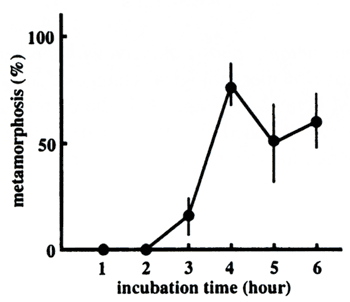 Figure 2. Time required for commitment to metamorphosis
Acropora tenuis planula larvae were treated with 1 x 10-6M Hym-248 for different periods of time and transferred to FSW, and the metamorphosis frequency was counted 12 hours after the beginning of the peptide treatment. The values are the average percentages of triplicates of each experiment (SE values are shown by bars).
Maturation time course of slick larvae
All the larvae in the collected slick seemed to be acroporids judged by their appearance; size, color and the absence of symbiotic algae. In order to know the culture period required to obtain mature larvae, which are competent for metamorphosis, the larvae collected from slicks were subjected to the metamorphosis assay with 2x10-6M Hym-248 along days of culture. The results are summarized in Figure 3. Larvae of the day 3 and 3.5 after fertilization did not respond to the peptide at all, whereas the metamorphosis frequency of the day 4 larvae exceeded 50 percent. The day 5.5 larvae revealed the 100% metamorphosis ability, and the high metamorphosis efficiency was kept till 6 days after fertilization. Further experiments were not done.
Figure 3. Metamorphosis ability of slick larvae during culture
Changes of the metamorphosis ability during culture were measured by treatment with 2x10-6M Hym-248. The metamorphosis percentages are the average of duplicates.
|