|
PRELIMINARY SELF-PRESERVATION EXPERIMENTS OF METHANE HYDRATE PELLETS
The authors have already obtained some meaningful results so far in the research project. From among them, introduced is the result of preliminary self-preservation experiments of pelletized methane hydrate, which were conducted to estimate their self-preservation property. The methane hydrate pellets (MHPs) tested in these experiments were made by MES, using methane hydrate production and pelletization trial machines. The photograph of the MHPS is shown in Figure 1. The MHP is a spherical body with the weight of approximately 3 grams and the diameter of 20 millimeters. Methane hydrate powder was synthesized in mixing method from water and pure methane gas (99.99% purity) at 5.2MPa to 5.4MPa and at the temperature lower by 2K to 7K than the methane equilibrium temperature corresponding to the pressure. After taken out from the mixer, the hydrate powder was kept in a Dewar vessel at roughly - 196℃ with liquid nitrogen. The hydrate powder was taken out and pelletized one at a time with a trial compressor at atmospheric pressure and at -20℃elsius. No extra heat and no ice were added during the pelletization process. Then, it is supposed that hydrate dissociation partly started and the pellet was a mixture of methane hydrate and ice. Thermal history of the hydrate powder/pellets was not controlled thoroughly, since the machine was developed for the main purpose of NGHPs' high-speed mass production. Manufactured MHPs had been kept in a Dewar vessel at roughly - 196℃ with liquid nitrogen again until they were used in the experiments.
 Figure 1. Photograph of methane hydrate pellets
In the experiments, weight change for one set of five MHPs was measured using an electronic balance (the minimum graduation: 1 milligram). Enclosed in a container with small holes, each MHPs' set was stored in a freezer that was kept at -20℃, -15℃, -10℃ and -5℃ respectively, in order to examine influence of temperature changes upon MHPs' self-preservation property. This temperature variation was specifically chosen to simulate the possible temperature fluctuations of the cargo hold during shipment (Ohta et al., 2002). The measurement period was set at three weeks, simulating a shipment time from natural gas fields in southeastern Asia to Japan, and taking into account storage on land. Shown in Figure 2 is the weight change of MHP's sets, assuming the weight ratio of each one at 'base time' as 100 percent. Here, 'base time' means the time when roughly one hour passed after the MHPs' set was picked out from the container cooled down by liquid nitrogen. As a result, it was confirmed that the lower the set temperature became from -5℃ to -20℃, the lower the shrinkage of the MHP's set became.
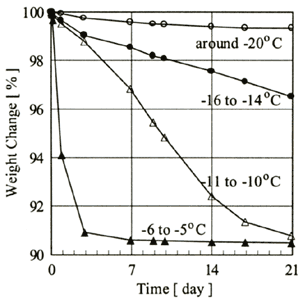 Figure 2. Weight change of MHPs' set
Promptly after the weight change was monitored for three weeks, the remaining MHPs' set was put at a normal temperature. The contained gas weight was measured by decomposing the set into water and methane gas completely, making sure that the water did not evaporate. Supposing that the weight decrease of the MHPs' set is equal to the weight of emitted methane gas, then the contained gas weight at every time can be calculated from the above weight change and the gas weight remained in the set after three weeks from the base time. From these data, dissociation rates of the MHPs' set were estimated. The MHPs were then kept at low temperature/humidity in order to prevent its weight from increasing owing to attached moisture in the air. Then, influence of sublimation was not taken into account.
The results of the contained methane gas measurement are shown in Table 1, and the estimated change of the hydrate ratio for every set temperature is shown in Figure 3. In Table 1, 'State I' means the state at the base time, and 'State II' means the state of the complete dissociation after 3 weeks from the base time. As seen from Figure 3, every MHP included 20% to 28% ice in at the base time. It is assumed that the ice had resulted from both imperfect conversion from water into hydrate in the hydrate synthesis process and part dissociation in the pelletization process. At this stage, it is not clear if the ice existed on the granular scale or as a rind encasing the entire pellet, or both.
Table 1. Results of contained gas measurement
| Set Temperature [℃] |
-5 |
-10 |
-15 |
-20 |
| Weight of MHPs' Set (State I) [gf] |
13.754 |
13.794 |
14.809 |
15.355 |
| Weight of MHPS' Set (State II) [gf] |
13.601 |
13.639 |
12.933 |
12.557 |
| Ratio of Contained Methane Gas [%] |
0.63 |
0.64 |
7.03 |
10.11 |
| Hydrate Ratio [%] |
4.7 |
4.8 |
52.5 |
75.4 |
|
Figure 3. Hydrate ratio change of MHPs' set
Both at -5℃ and -10℃, only approximately 5% of the theoretically contained gas remained in the MHPs' sets after a lapse of 3 weeks from the base time. On the other hand, at -15℃/-20℃, approximately 0.5 to 0.75 of the theoretically contained gas remained in the MHPs' sets even after a lapse of three weeks from the base time. It should be added that, the specific characteristics of the MHPs, used in these experiments such as contained methane gas ratio, are expected to be changed (improved) in the future. On the basis of the experimental data, average change rates of the hydrate ratio for three weeks from the base time at each temperature were calculated 47.9%/day at -5℃, 3.7%/day at -10℃, 2.6%/day at -15℃ and 0.5%/day at -20℃elsius. According to these results, it is qualitatively assumed that the dissociation rate of the MHPs' set used in the experiment decreased monotonically as the temperature around the set dropped. The complicated temperature-dependence of the self-preservation effect, which Stern et al. pointed out (2002), could not be observed in the experiment. It is inappropriate, however, to discuss the matter at this stage, since the temperature-pressure history in hydrate synthesis/pelletization process was not controlled fully with close attention in our experiments. The MHPs' set dissociated rather slowly at -20℃ in the experiments, so it is assumed that the MHP's self-preservation is maintained on the time scale including seaborne transportation. Namely, there is assumed to be every possibility of transporting NGHPs by ship from the viewpoint of hydrate's self-preservation.
CLOSING REMARKS
Regarding self-preservation, there are still a lot of problems to be solved for realization of the NGHP seaborne transportation, such as the estimates of (1) influence of pelletization upon self-preservation, (2) influence of pressure-temperature history in hydrate synthesis/pelletization process upon self-preservation, (3) self-preservation of NGHP (not MHP) (since the dissociation behavior of natural gas hydrate is assumed to be quite different from that of methane hydrate owing to the extremely different conditions required for thermodynamic stability), (4) mechanism of self-preservation effect and so forth. The authors are going to devote all our energies to tackling these problems continuously.
ACKNOWLEDGEMENT
This research is being conducted with financial support of the Corporation for Advanced Transport and Technology (CATT) in Japan.
REFERENCES
Gudmundsson, J.S., Parlaktuna, M., and Khokhar, A.A. 1994. Storing natural gas as frozen hydrate. SPE Production and Facilities, pp. 69-73.
Gudmundsson, J., and Borrehaug, A. 1996. Frozen hydrate for transport of natural gas, 2nd International Conference on Natural Gas Hydrates, pp.415-422.
Gudmundsson, J.S., Andersson, V., Levic, O.I., and Mork, M. 2000. Hydrate technology for capturing stranded gas, Gas Hydrate, Challenges for the Future, Annals of the New York Academy of Sciences, 912, pp.403-410.
Max, M. D., 2000, Chapter 18: Hydrate as a future energy resource for Japan, Natural Gas Hydrate in Oceanic and Permafrost Environments, Kluwer Academic Publishers, pp.225-238.
Nakajima, Y., Takaoki. T., Ohgaki, K. and Ota, S. 2002. Use of hydrate pellets for transportation of natural gas - II - Proposition of natural gas transportation in form of hydrate pellets -, Proceedings of the 4th International Conference on Gas Hydrates, pp.987-990.
Ota, S., Uetani, H. and Kawano, H. 2002. Use of hydrate pellets for transportation of natural Gas - III - Safety measures and conceptual design of natural gas hydrate pellet carrier -, Proceedings of the 4th International Conference on Gas Hydrates, pp.991-996.
Shirota, H., Aya, I., Namie, S., Bollavaram, P., Turner, D. and Sloan, E.D. 2002. Measurement of methane hydrate dissociation for application to natural gas storage and transportation, Proceedings of the 4th International Conference on Gas Hydrates, pp.972-977.
Sloan, E.D. 1998. Clathrate hydrates of natural gases, 2nd Ed, Marcel Dekker, New York.
Stern, L.A. et al., 2001, Anomalous preservation of pure methane hydrate at 1 atm, Journal of Physical Chemistry B, 105, pp.1756-1762.
Stern, L.A., Circone, S., Kirby. S. H., and Durham, W. B., 2002, New insights into phenomenon of anomalous or "Self" preservation of gas hydrates, Proceedings of the 4th International Conference on Gas Hydrates, pp. 673-677.
Takaoki, T., Iwasaki, T., Katoh, Y., Arai, T., and Horiguchi, K., 2002, Use of hydrate pellets for transportation of natural gas - I - Advantage of pellet form of natural gas hydrate in sea transportation -, Proceedings of the 4th International Conference on Gas Hydrates, pp.982-986.
Tamura, I., Kagajyo, T., Kuwabara, S., Yoshioka, T., Nagata, Y., Kurahashi, K. and Ishitani, H. 1999. Proceedings of 15th Energy System/Economics/Environment Conference. Japan Society of Energy and Resources, pp.419-423.
Yakushev, V.S. and Istomin, V.A. 1992. Gas-hydrates self-preservation effect, Physics and Chemistry of Ice, Hokkaido University Press, Sapporo, pp.136-139.
|