|
RESULTS AND DISCUSSION
SUL-I and SUL-II from the large flower-like globiferous pedicellariae of T. pileolus are D-galactose-specific lectins with molecular masses 32 kDa and 23 kDa, respectively (Nakagawa et al., 1996, 1999b). On the other hand, TGL-I from the small globiferous pedicellariae of T. gratilla is a heparin-specific lectin with a molecular mass of 23 kDa (Nakagawa et al., 1999a). SUL-I induced mitogenic stimulation on murine splenocytes in lower dose ranges such as 0.5 μg/ml. At higher doses SUL-I had an inhibitory effect on the splenocytes. However, SUL-II and TGL-I did not induce significant activity on the splenocytes as shown in Figure 2.
Figure 2. Comparison of mitogen response to SUL-I, SUL-II and TGL-I on murine
splenocytes
Further studies of biological activities of SUL-II and TGL-I are in progress
for comparison with those of SUL-I. More recently, we found that Contractin A, a mannose-containing glycoprotein
(18 kDa) (Nakagawa et al., 1991) is a novel lectin that causes smooth muscle contraction and relaxation.
Contractin A also induced mitogenic stimulation on murine splenocytes (data not shown). The dual response
to SUL-I was effectively inhibited by 50 mM D-galactose (data not shown). Thus, the data suggest that
SUL-I may exhibit mitogenic and inhibitory activities through binding to D-galactose containing carbohydrates
that are present on the surface of murine splenocytes. Moreover, it has been suggested that SUL-I binds
to D-galactose residues of Datura stramonium agglutinin (DSA) to interfere with mast cell activation
induced by DSA, a glycoprotein with arabinose and D-galactose residues (Suzuki-Nishimura et al., 2001).
Our previous finding revealed that SUL-I induces chemotactic activity of guinea-pig neutrophils (Nakagawa
et al., 1996).
In the present study, the chemotactic and phagocytic responses to SUL-I were
also examined on guinea-pig macrophages. SUL-I had chemotactic and phagocytic activities for guinea-pig
macrophages in dose dependent manner (Fig.3). In human polymorphonuclear leukocytes SUL-I exhibited chemotactic
activity (data not shown). Chemotaxis and phagocytosis by leukocytes play an important role in the defense
reactions to infection and injury in higher vertebrates. Thus, it is interesting that SUL-I as a chemoattractant
may be a useful tool for biomedical research. Sequence analysis of intact SUL-I indicated N-terminal sequence
from Ala-I to Ile-35 (Nakagawa et al., 1999b). SUL-I shows five glycine residues in the sequence region.
SUL-II was subjected to partial amino acid sequence analysis. The sequence of 21 residues from the N-terminal
was established. The N-terminal amino acid is serine. SUL-II is rich in serine (Table 1). Although SUL-II
did not show a sequence homology to SUL-I, it was found to be 45% and 40% homologous to the sequence of
Contractin A (Nakagawa et al., 1991) and UT841 from T. pileolus (Zhang et al., 2001), respectively.
SUL-II, Contractin A and UT841 may be a phospholipase A2-like substance, because
there is a good relationship to the amino acid sequence of phospholipase A2
(Takasaki et al., 1990).
On the other hand, SUL-I is related to the segment Tyr-Gly-Arg of the rhamnose-binding lectins (SAL and STL2) from fish eggs (Tateno et al., 1998; Hosono et al., 1999). Although physiological roles of multiple lectins from the toxopneustid sea urchins are not well understood, our data suggest an extracellular function for SUL-I and Contractin A that may have wide-ranging effects, and suggest that these lectins can be used as valuable tool for analysis of inflammation, differentiation and development of cells. Further structural studies on SULs, Contractin A and TGL-I are needed to elucidate the biological functions of sea urchin venoms.
| (Enlarge: 52KB) |
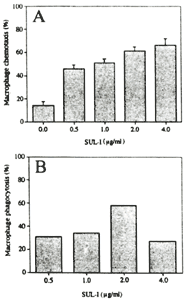 |
Figure 3. Effect of SUL-I on macrophage chemotaxis (A) and phagocytosis
(B)
Table 1. Comparison of partial amino acid sequence of sea urchin lectins
and fish lectins
| (Enlarge: 22KB) |
 |
ACKNOWLEDGMENTS
We would like to thank Professor K. Ohura and Dr. M. Shinohara, Osaka Dental University, for measuring chemotaxis and phagocytosis. We are also grateful to Professor Y. Tomihara and Dr. Y. Araki for their constant interest in this work, and Mr. H. Nada and Mr. H. Nagata for collection of sea urchins.
REFERENCES
Alender, C.B., G.A. Feigen and J.T. Tomita. 1965. Isolation and characterization of sea urchin toxin. Toxicon. 3(1):9-17.
Barondes, S.H., D.N.W. Cooper, M.A. Gitt and H. Leffler. 1994. Galectins. Structure
and function of a large family of animal lectins. J. Biol. Chem. 269(33):20807-20810.
Bradford, M.M. 1976. A rapid sensitive method for the quantitation of microgram
quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72:248-254.
Drickamer, K. 1988. Two distinct classes of carbohydrate-recognition domains
in animal lectins. J. Biol. Chem. 263(20):9557-9560.
Fujiwara, T. 1935. On the poisonous pedicellariae of Toxopneustes pileolus
(Lamark). Annot. Zool. Japan. 15(1):62-69.
Hosono, M., K. Ishikawa, R. Mineki, K. Murayama, C. Numata, Y. Ogata, Y. Takayanagi
and K. Nitta. 1999. Tandem repeat structure of rhamnose-binding lectin from catfish (Silurus astotus)
eggs. Biochem. Biophys. Acta. 1472:668-675.
Kasai, K. and J. Hirabayashi. 1996. A family of animal lectins that decipher
glycocodes. J, Biochem. 119(1): 1-6.
Kimura, A., H. Hayashi and M. Kuramoto. 1975. Studies of urchin-toxins: Separation,
purification and pharmacological actions of toxinic substances. Japan. J. Pharmacol. 25(2):109-120.
Laemmli, U.K. 1970. Cleavage of structural protein during the assembly of the
head of bacteriophage T4. Nature. 227(259):680-685.
Mendes, E.G., L. Abbud and S. Umiji. 1963. Cholinergic action of homogenates
of sea urchin pedicellariae. Science. 139(3553):408-409.
Mikheyskaya, L.V., E.V. Evtushenko, R.G. Ovodova, N.I. Belogortseva and Y.S.
Ovodov. 1995. Isolation and characterization of a new β-garactose-specific lectin from the sea worm Chaetopterus
variopedatus. Carbohydr. Res. 275:193-200.
Nakagawa, H., A. Tu and A. Kimura, 1991. Purification and characterization
of Contractin A from the pedicellarial venom of sea urchin, Toxopneustes pileolus. Arch. Biochem. Biophys.
284(2):279-284.
Nakagawa, H., T. Hashimoto, H. Hayashi, M. Shinohara, K. Ohura, E. Tachikawa
and T. Kashimoto. 1996. Isolation of a novel lectin from the globiferous pedicellariae of the sea urchin
Toxopneustes pileolus. Adv. Exp. Med. Biol. 391:213-223.
Nakagawa, H., C. Yamaguchi and H. Hayashi. 1997. Biologically active substances
from sea urchins. J. Natural Toxins. 6(2):193-202.
Nakagawa, H., C. Yamaguchi, H. Sakai, K. Kanemaru and H. Hayashi. 1999a. Biochemical
and physiological properties of pedicellarial lectins from the toxopneustid sea urchins. J. Natural
Toxins. 8(3):297-308.
Nakagawa, H., C. Yamaguchi, F. Tomiyoshi and H. Hayashi. 1999b. A novel mitogenic
lectin from the globiferous pedicellariae of sea urchin, Toxopneustes pileolus. Jour. Chem. Soc. Pak.
21(3):305-310.
Ohura, K., M. Shinohara, K. Ogata, A. Nishiyama and M. Mori. 1990. Leucocyte
function in rats with naturally occurring gingivitis. Archs. Oral Biol. 35 Suppl.:185s-187s.
Ozeki, Y., T. Matsui and K. Titani. 1991. Amino acid sequence and molecular
characterization of a D-galactose-specific lectin purified from sea urchin (Anthrocidaris crassispina)
eggs. Biochemistry. 30(9):2391-2394.
Ozeki, Y., E. Tazawa and T. Matsui. 1997. D-galactoside-specific lectins
from the body wall of an echiuroid (Urechis unicinctus) and two annelids (Neanthes japonica and Marphysa
sanguinea). Com. Biochem. Physiol. 118B(1):1-6.
Suzuki-Nishimura, T., H. Nakagawa and M.K. Uchida. 2001. D-galactose-specific
sea urchin lectin sugar-specifically inhibited histamine release induced by Datura stramonium agglutinin:
Differences between sugar-specific effects of sea urchin lectin and those of D-galactose- or L-fucose-specific
plant lectins. Japan Journal of Pharmacology. 85(4):443-452.
Takasaki, C., F. Yutani and T. Kajiyashiki. 1990. Amino acid sequences
of eight phospholipase A2 from the venom of Australian king brown snake, Pseudechis
australis. Toxicon. 28(3):329-339.
Tateno, H., A. Saneyoshi, T. Ogata, K. Muramoto, H. Kamiya and M. Saneyoshi.
1998. Isolation and characterization of rhamnose-binding lectin from eggs of steelhead trout (Oncorhynchus
mykiss) homologous to low density lipoprotein receptor superfamily. J. Biol. Chem. 273(30):19190-19197.
Yokosawa, H., K. Harada, K. Igarashi, Y. Abe, K. Takahashi and S. Ishii.
1986. Galactose-specific lectin in the hemolymph of solitary ascidian. Halocynthia roretzi. Molecular,
binding and functional properties. Biochem. Biophys. Acta. 870:242-247.
Zhang, Y., J. Abe, A. Siddiq, H. Nakagawa, S. Honda, T. Wada and S. Ichida.
2001. UT841 purified from sea urchin (Toxopneustes pileolus) venom inhibits time-dependent45Ca2+
uptake in crude synaptosome fraction from chick brain. Toxicon. 39(8):1223-1229.
|