PRESENTATION OF RESULTS OF THE VIETNAM RDF PROJECT STUDY: SUMMARY
UNIVERSITY OF TOKYO AND MANAGEMENT SCIENCES FOR HEALTH
Doi Moi and the Context of the Project
A wide network of health centers was designed around the commune cooperative system. Of the 9,929 communes in the country,
・ 9,243 (93.1%) have commune health centers (CHCs),
・ 600 (6.1%) have no CHCs but have resident commune health workers (CHWs),
・ only seventy-eight (0.79%) have no commune-based health services, and
・ on average each CHC is staffed by three to five CHWs with at least one assistant doctor and a nurse midwife.
Prior to 1986 there were in the villages brigade nurses who were supported by their production brigades. Since the introduction of the market economy as a result of the “Doi Moi Renovation" in 1986, the cooperative system has collapsed. This led to the dismantling of production brigades and the collapse of the brigade nursing system. However, the CHCs endured, as there were physical structures that had to be run and maintained. The ownership of CHCs was thrust upon the commune people's committees. However, from the financial and management perspectives, the commune people's committees were ill-equipped and unprepared to run the CHCs. Supporting the CHC infrastructure has placed a severe strain on the health budget. Though 9,243 communes have CHCs, 686 do not. An overwhelming number of communes without CHCs (445) are in the northern mountainous region.
There are major tasks that need to be addressed to further develop the health care system:
・ Provision of policy directives on cost recovery, financing and facility management
・ Adequate provision of drugs and equipment
・ Appropriate training and continuing education for health workers
・ Improved wages and remuneration for health workers
・ Coordination of services
Background for the Study
・ The overall goals and aims of Nippon Foundation support were well stated.
・ Specific and measurable objectives with indicators and baseline measures that would track progress toward achieving these objectives were, however, not explicitly included in the core project documents.
・ UNICEF, as the implementing partner, developed an annual plan of action that included operational objectives for the year.
・ In Vietnam, a baseline survey to assess the situation was carried out.
・ The extent to which the information collected and the results and conclusions were employed in the planning, implementation and monitoring of the project was not clear.
・ Some form of monitoring had been done and various reports concerning the implementation of the Essential Drug Project were submitted.
The Vietnam project has reached a crucial turning point.
Accordingly, assessing the impact of project activities since its inception and the finding of appropriate indicators and relevant baseline information about the performance of the RDFs emerged as a critical need. The present study was designed to provide the necessary information to all involved partners, to improve project management and implementation during the next phase and also to assist the Nippon Foundation and other interested donors to determine the nature and future direction of their support.
Goals of the NF Project in Vietnam
・ Ensure sufficient supply of drugs at participating commune health centers
・ Encourage community participation in the provision of public health services through payment for drugs, user charges and co-management of RDFs
・ Improve commune health worker's capability to manage RDFs in financial and inventory terms
・ Improve quality of health care, equity and utilization of commune health centers
Questions to Be Answered by the Study
・ How has the project developed over time?
・ What factors-such as MOH and government policies, economic and social changes, changes in the policy and practice of international organizations-have influenced the implementation of the project?
・ What problems have arisen in the course of project implementation and how were these addressed?
・ How can performance be measured to assess its impact?
・ How can performance be further improved?
・ What improvements need to be made in the management and administration of the project?
・ How realistic would it be to replicate, scale up or incorporate the project into overall government services and make it self-financing?
CHRONOLOGY OF THE NIPPON FOUNDATION RDF PROJECT
| 1986 |
Doi Moi Reform inaugurated |
| 1989 |
Bamako Initiative started and drug user charges introduced |
| 1991 |
Acute shortage of drugs recognized |
| 1992 |
Support for drugs and pharmaceutical sector development solicited |
| 1993 |
Support of US$2 million per annum for three years to MOH Vietnam and UNICEF proposed by NF, which asked to develop program with MOH |
| 1994 |
In response to UNICEF request, technical assistance of US$300,000 per annum for three years approved by Nippon Foundation for baseline surveys (50,000), training (150,000) and social mobilization (100,000) |
| 1995 |
First batch of drugs procured through UNIPAC and sent to MOH, destined for all communes in all districts in eight provinces selected by MOH/UNICEF |
| 1995 |
Program review carried out by the University of Tokyo's Department of Health Policy & Planning and the Nippon Foundation |
| 1996 |
Review/evaluation of the RDF/Bamako Initiative initiated and funded by MOH |
| 1996 |
Second batch of Nippon Foundation drugs procured through a Japanese procurement agency and sent to MOH Vietnam for a second lot of eight districts |
| 1997 |
Formal study of the RDF project (MOH/UNICEF/MSH/University of Tokyo) |
Seed stock procurement and distribution:
・ The first batch of seed stock was purchased through UNICEF/UNIPAC and then shipped to the MOH. Vinapharm received the consignment and distributed it using commune population as the basis. This was done through provincial and district health services.
・ The batch of seed stock was purchased through a Japanese procurement agency and then shipped to the MOH. Vinapharm, as before, was responsible for distribution to the second phase provinces.
TIMELINE OF EVENTS 1986-1997
| Event/Activity |
1986 |
1987 |
1988 |
1989 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
| Doi Moi Initiative |
○ |
○ |
○ |
|
|
|
|
|
|
|
|
|
| User charges introduced |
○ |
|
|
|
|
|
|
|
|
|
|
|
| National health insurance started |
|
|
|
○ |
|
|
|
|
|
|
|
|
| Bamako Initiative started in pilot districts |
|
|
|
|
○ |
|
|
|
|
|
|
|
| Drug user fees introduced in BI districts |
|
|
|
|
○ |
|
|
|
|
|
|
|
First major review of the National Essential
Drugs List (EDL) |
|
|
|
|
|
○ |
|
|
|
|
|
|
| Vinapharm changes from union to association |
|
|
|
|
|
○ |
|
|
|
|
|
|
Clinical pharmacology introduced at College of
pharmacy |
|
|
|
|
|
|
○ |
|
|
|
|
|
| World Bank health sector review |
|
|
|
|
|
|
○ |
|
|
|
|
|
| Nippon Foundation RDF Project conceived |
|
|
|
|
|
|
|
○ |
|
|
|
|
Salaries for CHWs in mountainous areas
authorized by national government |
|
|
|
|
|
|
|
○ |
|
|
|
|
Authorization to open and operate private
clinics and phrmacies |
|
|
|
|
|
|
|
○ |
|
|
|
|
2nd SIDA project on health sector
development induding pharmaceuticals |
|
|
|
|
|
|
|
|
○ |
|
|
|
Nippon Foundation RDF Project technical
assistance funds released |
|
|
|
|
|
|
|
|
○ |
|
|
|
| Salaries for CHWs in lowland areas authorized by national government |
|
|
|
|
|
|
|
|
|
○ |
|
|
| Nippon Foundation RDF Project drug seed stock received |
|
|
|
|
|
|
|
|
|
○ |
|
|
Nippon Foundation RDF Project training begins
(late in the year) |
|
|
|
|
|
|
|
|
|
○ |
○ |
|
| National government authorizes joingt venture private hospitals |
|
|
|
|
|
|
|
|
|
○ |
|
|
| National Drug Policy Review and 2nd review of the national EDL |
|
|
|
|
|
|
|
|
|
|
○ |
|
| Drug Administration of Vietnam formed out of the Dept of Pharmacy |
|
|
|
|
|
|
|
|
|
|
○ |
|
First National Conference to review and
reconcile national health policies held |
|
|
|
|
|
|
|
|
|
|
○ |
|
| Vinapharm becomes a corporation |
|
|
|
|
|
|
|
|
|
|
○ |
|
| MOH strategy focusing on the poor initiated |
|
|
|
|
|
|
|
|
|
|
○ |
|
MIS Project to integrate various project
information systems started |
|
|
|
|
|
|
|
|
|
|
○ |
|
HIV/AIDS Control Committee brought under
the General Committee |
|
|
|
|
|
|
|
|
|
|
|
○ |
Dept of Borderland Disease Control formed
within MOH |
|
|
|
|
|
|
|
|
|
|
|
○ |
Decree authorizing formation of hospital
therapeutic councils promulgated |
|
|
|
|
|
|
|
|
|
|
|
○ |
Official government approval for the charging
of drugs fees in RDF project areas |
|
|
|
|
|
|
|
|
|
|
|
○ |
GMP certification for three Vietnamese drug
factories granted |
|
|
|
|
|
|
|
|
|
|
|
○ |
| Dept. of Legislation formed within MOH |
|
|
|
|
|
|
|
|
|
|
|
○ |
| National Health Insurance review initiated |
|
|
|
|
|
|
|
|
|
|
|
○ |
| User charges revised to refiect actual costs |
|
|
|
|
|
|
|
|
|
|
|
○ |
| Re-introduction of government subsidy for drugs in SDAs |
|
|
|
|
|
|
|
|
|
|
|
○ |
| CHW salaries provided from provincial level |
|
|
|
|
|
|
|
|
|
|
|
○ |
| Dept. of planning and Finance splits into two departments |
|
|
|
|
|
|
|
|
|
|
|
○ |
From 1986 to 1994, there was a notable shift from the provision of free care to partial/full cost sharing. From the launching of the Doi Moi Initiative in 1986 till the start of the implementation of the Nippon Foundation RDF project in 1994, a number of key events occurred. These were:
・ introduction of user charges at health facilities,
・ implementation of the Bamako Initiative and the institution of drug fees to recover full costs in Bamako Initiative districts,
・ significant change in the role of Vinapharm from being a government entity to becoming a semi-autonomous Association of Pharmaceutical Enterprises,
・ authorization for the opening of private pharmacies and clinics,
・ authorization for salaries to be paid to commune health workers in mountainous areas where access to health care is very poor both in terms of available infrastructure and the purchasing power of rural people, and
・ initiation of the Nippon Foundation project to systematically increase access to essential, affordable and acceptable quality drugs.
The momentum of change was slow and the events were spread out over almost a decade. During this period, the assumptions underlying the Doi Moi Initiative gradually permeated the service sectors allowing for the cost sharing principle to become the norm rather than the exception. The MOH conceded that there were acute shortages in the areas of pharmaceutcals, medical equipment and quality of care and began to develop an approach to address these.
The basis of increased donor participation in the development of the health sector and in the consolidation of gains made during the pre-Doi Moi period was also put in place during this time.
From 1994 to 1996, the pace of change in policy development and structural reorganization within the Ministry of Health and the Government of Vietnam was astonishingly rapid. Key events that occurred during these two years include:
・ authorization of salaries for all CHWs to improve provision of care at the commune level and to make commune health workers more accountable,
・ provision of incentives for staff to carry out preventive activities at the CHCs instead of just providing curative services and using the CHSs to buttress existing private practices,
・ systematic implementation of the Nippon Foundation RDF project and its expansion into the second phase now covering seventeen provinces,
・ authorization to set up joint venture hospitals and clinics,
・ national review of the Vietnam Drug Policy and Essential Drugs List (EDL),
・ remarkable shift within the MOH and government, from a pharmaceutical production focus to a regulation and control focus; improvement in the monitoring of the pharmaceutical sector made evident through the conversion of the Department of Pharmacy to Drug Administration of Vetnam,
・ evolution of Vinapharm into a corporate entity with full autonomy regarding the operations of the corporation,
・ increased emphasis on equity through the drafting of a national strategy to focus on the poor in view of the consequences of new market mechanisms within the health sector, and
・ initiation of the review and revision of the Management and Health Information Systems.
Around this time, other donors also started to take an increasing interest in supporting the health sector as a result of the development of a health sector support proposal by the World Bank, the intensification of SIDA support, more focused UNICEF and UNDP support, closer collaboration between donors and the Ministry of Health, and close scrutiny by the MOH of its own performance and the reorganization required to change the rhetoric of PHC into reality.
During the first half of 1997, the momentum for change continued to accelerate with the following events occurring:
・ Decree authorizing the formation of therapeutic councils in hospitals issued to promote rational drug use at the hospitals
・ Formal government approval to officially charge fees in the Nippon Foundation RDF project and Bamako Initiative provinces
・ GMP certification granted to two Vietnamese pharmaceutical factories
・ National Health Insurance review initiated to revise the premium and reimbursement schedule
・ Review of user charges for adjustments to reflect actual costs
・ CHW salaries made the responsibility of provincial level governments
・ Reintroduction of government subsidies for essential drugs in the SDAs
The MOH has recognized that with the rapidly developing pharmaceutical sector, growing at an average rate of 10% per annum, and the increasing awareness among the general population about health care quality, it needs to reform and reorganize in order to respond more adequately to the new challenges, particularly with regard to the costs of providing health care and the improvement of the quality of care. Therefore, during 1997, the momentum of change within the MOH has accelerated and significant changes in the structure of the Ministry are being initiated. That there is no longer a shortage of drugs appears to have shifted the focus from resolving shortages and increasing access to essential drugs to promoting rational drug use and improving the quality and equity of care. It is expected1 that the pace of change will continue to intensify and expand in scope. It will include expansion and the scaling-up of the RDF scheme but with the inclusion of rational drug use, increased community co-management of health services rather than just RDFs, and strengthening management capacity at the district and commune levels through more effective implementation of decentralization. The role of international donors in assisting the development of health care in Vietnam and the coordinating role of the MOH will also expand, providing new opportunities and presenting new challenges.
1 Source: personal communication from Dr. Duc An, Director, Department of Planning, Ministry of Health of Vietnam (August 1997)
DRUG SUPPLY SYSTEM IN VIETNAM
Development of the Pharmaceutical Sector
In the pharmaceutical sector,2 there are:
2 Source: Pharmaceutical Department, Ministry of Health of Vietnam (1996)
17 central pharmaceutical enterprises
118 local pharmaceutical companies and factories
18 licensed pharmaceutical investment projects and joint-ventures
170 private pharmaceutical enterprises, limited and stock companies.
>7000 private retail pharmacies
・ Drug imports in 1996 amounted to US$349,409,000 and grew by 25% from 1995 (US$279,527,000). It grew 23% over 1995 at the central level and 26% at the local level.
・ The sale of pharmaceuticals in 1996 amounted to VND 4,721,634 million and grew by 46% over sales in 1995 (VND 3,244,369 million). This was a 49% increase at the central level and a 45% at the local level.
・ The per capita drug expenditure was US$4.6 in 1996 as compared to US$4.2 in 1995, an increase of 9% in one year.
Problems Encountered during Project Implementation
・ RDFs were seen as another vertical project
* Current location of the RDF project
* RDFs are now seen as an entry point for the development of PHC and the health care system
・ Provision of seed stock
* Seed stock provided during first phase as requested, included items not useful at the commune level
* Local pricing of NF seed stock resulted in reduction of the value of the seed stock
・ RDF guidelines developed but no standardized procedures and forms for operating and managing RDFs, community co-management, reporting and supervision
・ Initially developed training materials were inappropriate and required major revisions resulting in delays
・ Supervision and monitoring of RDFs not integrated into routine supervision from the district level for reasons such as:
* Vertical orientation of the RDF project
* Lack of resources for monitoring and supervision
* Lack of appropriate indicators for monitoring
* Lack of systematic routine supervision from the district level.
* Limited authority over CHCs with particular reference to administrative/fiscal matters
・ Irrational drug use
・ Quality assurance of locally manufactured and imported drugs because of poor regulation and control
・ Large gap between policy and implementation because of policy conflicts due to piecemeal and uncoordinated development of policies related to essential drugs and their use at various levels
・ Ineffective coordination within the MOH and of donor-assisted projects
Preliminary Conclusions
・ Continuous availability of drugs in the rural areas is now a reality in the project communes through the setting up of RDFs but the cost, type, range and quality of drugs is still a concern.
・ The expanding private sector in terms of private pharmacies and private practitioners has made access to drugs easier but there is also an increase in irrational drug use and poly-pharmacy.
・ The expanding private sector has increased the range and type of drugs available in areas of major population concentrations, but it has also increased costs and the need for more extensive regulation and control, particularly in peripheral and remote areas.
・ Almost all public sector health facilities from the commune level up have operational RDFs. However, the orientation of these RDFs is different in different areas. The predominant orientation is the maintenance of the RDFs and the strengthening of it, so that with higher turnover, more of profits could then be used for the maintenance of the health facilities.
・ There is a clear demarcation emerging between the regulatory and drug control activities and the drug production activities of the MOH. The emergence of Vinapharm as a corporation, the recent formation of the Drug Administration of Vietnam, and the decree on the formation of therapeutic councils in all hospitals clearly indicates the movement of the MOH toward regulation and control and away from production. This means that capacity at the peripheral levels needs to be further strengthened and strengthened rapidly.
MOH DRUG AND REGULATION SYSTEM
PREPARED BY MOHAN NARULA, MARIA MIRALLES, TOMOKO FUJISAKI, WITH THE ASSISTANCE OF MOH AND UNICEF
(拡大画面: 78 KB)
RELATIONSHIP BETWEEN DRUG ADMINISTRATION, DEPARTMENT OF INSPECTION AND VINAPHARM
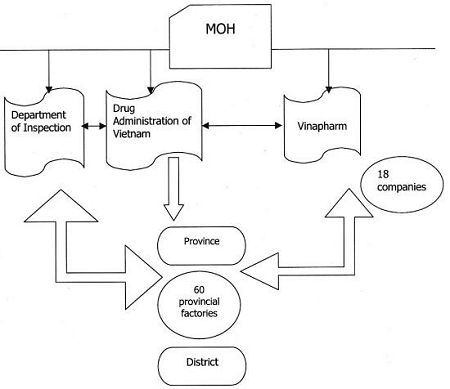
・ Decentralization at the political level appears to be in place but it is still not reflected in the service agencies where the centralized approach still appears to prevail. The capacity of the districts to plan, implement, supervise and monitor needs to be greatly enhanced if the quality of care is to be improved and the gains made by Vietnam not lost. The importance of regular monitoring and supervision of RDFs has been highlighted by the widespread implementation of RDFs at the commune level.
・ The development of procedures to improve the implementation of RDF guidelines with regard to the operation of the RDFs has now become a critical requirement.
・ Community health education on the proper use of medicines must now be made an integral part of the RDF project. It should involve health workers at CHCs.
・ Strengthening the management capacity of district and commune level health teams through a systematic training program is emerging as an urgent need. The training could start with TOTs at the national and provincial levels, and then lead to the training of selected district and commune level staff. The training could be built into the overall in-service and supervisory system of the MOH.
RDF Performance Indicators
1. Drug availability and drug pricing
A finding from this study is that for the twenty tracer drugs selected, although availability was slightly better in comparison groups at all levels, the relative difference between availability in the DPS and CHC groups was approximately the same (16%). However, with no other information on drug availability prior to the Nippon Foundation study project, it is not possible to determine if drug availability has improved or declined since inception of the project. What is clear, though, is that there is a greater availability of the same set of tracer drugs in the private sector.
Similarly, prices to consumers in the study and comparison groups were on average higher than prices offered in the private drug outlets. These products, however, are typically not generic and are not necessarily on the National Essential Drugs List. The expanding role of Vinapharm, the para-statal pharmaceutical company, should be evaluated in this light. With the new management of Vinapharm, together with recent approbation of RDFs by the central government, access to and availability of generic, essential medications at competitive prices may be further improved.
2. Utilization
According to the World Bank and to key informants, there has been an increase in the utilization of public sector services since 1995. This is most likely due to a combination of factors including the return of health care professionals to the public sector and to efforts to improve incentives (or wages). The perceived quality of care, it is suggested, has improved.
Utilization may have improved with successful RDFs because of the perception that the quality of public sector medicines is good. The targeted populations of the CHCs with limited resources are likely to feel satisfied with the quality of drugs supplied by RDFs. Indeed, there were few reports by the CHCs of poor quality drugs. During the period of declining public health services, a sharp increase in self-medication meant that people were spending a significant amount of household income on drugs available in the private sector. This was at a time when private pharmacies were expanding rapidly while the Ministry of Health had extremely limited capacity to conduct inspections.
The CPCs and district and provincial health authorities have continued to express interest in RDFs and, in particular, in learning how to expand them to meet unmet demand. Suggestions included finding additional seed stock or capital. Other alternatives for some RDFs may be a re-evaluation of their pricing strategies and certainly for most, tighter accounting and record keeping methods.
3. Rational drug use
Among the activities UNICEF has been promoting through the Nippon Foundation support for RDFs is the training of health workers on rational drug use. This is a very responsible and reasonable activity to accompany the provision of seed stock.
This study provides the baseline for a follow-up study that may be undertaken in the future.
The findings indicate little difference between the study and comparison groups on standard indicators of average number of drugs prescribed per encounter, and the percentage of antibiotics and injectables prescribed. There is some evidence that the practitioners in the study group were less likely to prescribe potentially inappropriate combinations of antibiotics than those in the comparison group.
The Ministry of Health is well on the way to assuring improved drug information, prescribing reviews and treatment guidelines for the inpatient setting. Similar efforts for the outpatient setting would be beneficial.
4. Financial performance
RDFs are one model for community-based drug cost recovery. This model has been applied in Vietnam for several years and has met with some success or it would not have endured. As record keeping of RDF activity has not been uniform, universal or transparent, the data collected was incomplete and unreliable. Despite these limitations, some definitive results were obtained measuring the performance of RDFs.
RDF performance is often measured only by the number of times a fund is able to revolve, and by relatively simplistic, unidimensional measures of financial well-being. In this study, measures typically used in the private sector allowed for a richer understanding of how well CHCs are able to manage the resources at their disposal and generate revenues.
Most of the RDFs in both the study and comparison groups showed both positive and negative signs in financial performance. A careful case by case evaluation could help individual RDFs to identify weaknesses and find ways to eliminate or overcome these.
The recent sanctioning of RDFs by the Vietnamese government is likely to give a boost to their performance as it provides explicit guidelines on what is expected from RDFs in terms of services and accountability.
Issues for Discussion
・ The MOH is particularly concerned about the issue of equity as fifteen million Vietnamese are officially considered below the poverty line. Therefore, with the current focus on the poor, mechanisms for providing exemptions and subsidies, particularly to specially disadvantaged areas (SDAs), are a priority.
・ In the pharmaceutical sector, the priority issue is to strengthen the MOH's ability to monitor, regulate and control the sector and to increase training in rational drug use. There are about 9000 items in the market with 600 active substances, making regulation and control a big and complex task.
・ The regulation and control of the drug imports, including extension of GMP status beyond the two factories in Vietnam, is also a high priority.
・ The upgrading of the quality of care at district hospitals is also a top priority, and in this area, the support of ADB and the World Bank will be forthcoming. The WB Rural Health Project is in the final stages of the approval process.
・ The Blood Transfusion Service needs to be expanded as the increasing spread of HIV and drug abuse requires improved screening of blood.
・ The RDF projects will be scaled up but in a phased manner starting with the SDAs and employing clear criteria.
・ Despite the fact there are enough drugs in the system, (public and private) distribution of these drugs is still uneven, especially in the SDAs.
・ Coordination of the various projects involved with essential drugs, rational drug use, RDFs and other pharmaceutical-related activities is poor despite the initiative taken by SIDA and the support of the MOH which has assigned this task to a vice minister. There has been very slow progress in coordinating donor projects.
・ There is an ineffective drug information system in the country. A drug information unit has been set up in the Hanoi College of Pharmacy but it seems not to be making any difference.
・ There does not currently exist a coordinated approach to drug supply and use issues. The recent transformation of the Department of Pharmacy into the Drug Administration of Vietnam has not resulted in any change in terms of coordination between the various departments and functions. These are still in the early stages.
・ There is and absence of a unified approach on the part of UN agencies (they tend to promote their own programs), NGOs and other bilateral donors involved with health care and drugs.
・ The situation has changed enough in the last five years that the availability of drugs is no longer the issue. The concerns are now RDU, quality of care, and equitable health care.
・ Supervision and Training
The system of supervision needs to be further developed. The present system needs to be systematized. Supervison by the district of the commune level needs to be strengthened and should include RDFs. The capacity of provinces to supervise their districts also needs to be strengthened.
・ Procedures and guidelines for RDFs in the following areas need significant revision and further development:
Record keeping
Finances
Inventory
Rational drug use
Decentralization
Community participation